
EAS Alumni Magazine October 2023 — Using geochemistry to understand the Earth
Three young professors in Cornell’s Department of Earth and Atmospheric Sciences are filling in some of the details of exactly how the basic materials of the planet move through the Earth system.
By Chris Dawson
With the exception of materials brought here by meteorites, comets, asteroids, and space debris, all of the “stuff” on Earth today has been here since the planet formed billions of years ago. The atoms that make up this “stuff” may not be in the same configurations as they were then, but they are all still here. Earth is a mostly-closed system and, as such, it is a master at reusing and recycling what is has available.
Researchers have made remarkable progress in the past 50 years describing the broad strokes of how the materials that make up rocks—the basic building blocks of the planet—cycle through the Earth system.
Three young professors in Cornell’s Department of Earth and Atmospheric Sciences are filling in some of the details between these broad strokes and, in the process, making some surprising discoveries. All three focus on geochemistry, but each is elucidating a different part of the cycle.
Megan Holycross recently published a paper investigating the processes that produce continents—features that are unique to our home planet. Esteban Gazel came out with a paper in 2023 showing that carbon dioxide quickly coming out of solution can drive magma from deep within the mantle all the way to the Earth’s surface. Nicole Fernandez discovered in the summer of 2023 that wildfire smoke from Canada was unexpectedly creating spikes in the phosphorus concentration in Tompkins County streams and creeks.

Making rocks in the lab
Assistant Professor Megan Holycross uses controlled laboratory experiments to develop new geochemical tools to quantify the rates and conditions of magmatic and metamorphic processes. Areas she and her lab group are interested in include calibrating new “crystal clocks” to time volcanic processes, quantifying the recycling of trace element in subduction zones, and developing new synchrotron spectroscopy analytical techniques.
A particular focus lately has been trying to understand the processes that influence the compositions of magmas that form and collect below continental arc volcanoes. This magma is what makes the rocks that comprise the continental crust. Continental arc magmas are depleted in iron and contain more oxidized iron compared to magmas that form the ocean crust. Consequently, the crust arc magmas form is lighter (less dense) than oceanic crusts and therefore rises above sea level—creating dry land.
 Currently, there are no methods available to visit a magma chamber below a continental arc volcano to see what is happening there—or even simply to grab a few samples. When Holycross needs a rock sample to examine, she goes into her lab on the first floor of Snee Hall, cranks up her Deltech controlled-atmosphere furnaces or her Rockland piston cylinders and makes her own rocks.
Currently, there are no methods available to visit a magma chamber below a continental arc volcano to see what is happening there—or even simply to grab a few samples. When Holycross needs a rock sample to examine, she goes into her lab on the first floor of Snee Hall, cranks up her Deltech controlled-atmosphere furnaces or her Rockland piston cylinders and makes her own rocks.
Over the past couple of years, she has shown a preference for creating garnets, and it is not because garnets are especially valuable. Garnets come in many varieties, but all have a silicate (SiO4) molecule attached to two other elements. The variation in garnets comes from the variation in those other two elements and leads to a wide range of colors. But it is not the variety of attractive colors, either, that drove Holycross to make them in her lab.
Rather, it was the popular hypothesis that continental crusts are lighter because as garnets crystallize in magma chambers deep below the surface they incorporate large amounts of unoxidized iron. Using her piston cylinder experiments, Holycross and her collaborators were able to show that garnets formed from molten rock at pressures and temperatures mimicking the conditions of a magma chamber had not incorporated enough unoxidized iron to explain the levels of iron-depletion and oxidation present in the magmas.
Holycross and her main collaborator on the project, the Smithsonian’s Elizabeth Cottrell, published their results in the journal Science in May in a paper titled “Garnet crystallization does not drive oxidation at arcs.” Which leads to the obvious question: “Well then…what DOES drive oxidation at arcs?”
This question is an important one to answer and it will guide a significant portion of Holycross’s work in the coming years.
But figuring out why continental crust is lighter than oceanic crust is not the only question Holycross is interested in. She has also played an important role in a research project led by Buz Barstow, assistant professor of biological and environmental engineering in Cornell’s College of Agriculture and Life Sciences. Barstow is looking for environmentally friendly ways to mine rare-earth elements using microbes.
In order to test the efficiency of the engineered microbes at releasing rare-earth elements from ores, it is essential to start with ores that have a known concentration of rare-earth elements in them. This is where Holycross and her furnaces come in. Holycross in collaboration with Gazel and Barstow was able to produce monazite crystals whose composition was known precisely. Barstow’s team then exposed those monazite crystals to bio-leaching compounds created by an engineered strain of Gluconobacter oxydans.
The team has had great success and their results are promising enough that one of Barstow’s postdoctoral researchers and a graduate student have spun off a start-up called REEgen to commercialize the process.
Also, Marc Fortin, a former postdoc (now at Corning, Inc) that worked with Gazel and Holycross produced synthetic lava in her laboratory furnaces as part of a study replicating possible exoplanet surfaces.
Deep Earth detective
Rather than making his own rocks in the lab, Esteban Gazel, the Charles N. Mellowes Professor in Engineering, collects samples of materials ejected from volcanoes and uses them to piece together the puzzle of where the material came from and what happened to it before it got to the surface. His lab includes advanced microscopy, spectroscopy, and spectrometry equipment that allows him to better understand and explain the Earth’s geochemical cycles.
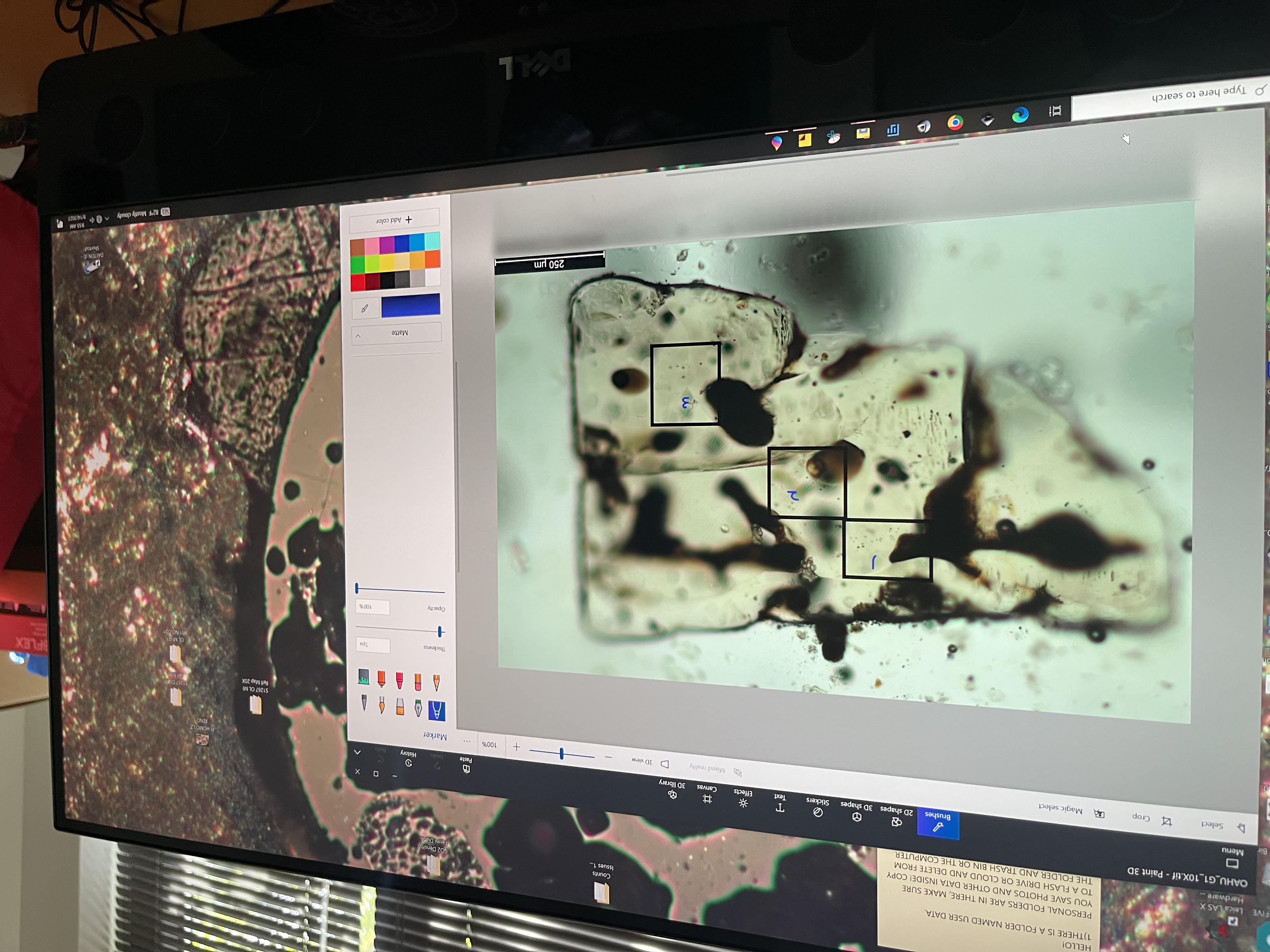
To get a sense of the depth and breadth of his research interests, it is helpful to take a look at a few of the papers Gazel has published in just the past year. In one study, Gazel and doctoral student Kyle Dayton examined carbon-dioxide-rich fluids trapped in olivine crystals ejected from the Cumbre Vieja volcano in the Canary Islands. Using precise Raman spectroscopy methods developed by Gazel’s lab team, they were able to determine the depth at which the magma in the eruption had been stored. They were able to improve the precision of these depth calculations by an order of magnitude, reducing errors from the kilometer range to the meter range and measuring samples as small as one micrometer.
This work is important because a fundamental question for volcanologists and risk management is exactly where magma is stored in the Earth’s crust and mantle. “That location matters because you can gauge the risk of an eruption by pinpointing the specific location of magma, instead of other signals like the hydrothermal system of a volcano,” Gazel said.
Using a high-precision carbon dioxide densimeter developed by Gazel lab team past doctoral student Charlotte DeVitre ’22, showed that basaltic volcanoes that are usually located in the interior of tectonic plates are fed by magma located much deeper in the mantle than previously thought—as deep as 20 to 30 kilometers below the surface.
Volcanologists have long believed that water is the driving force behind many explosive volcanic eruptions. Gazel showed that there is a different mechanism at work. His lab data support the idea that the exsolution of carbon dioxide from magma stored in the mantle drives the magma up through the lithosphere and controls volcanic explosivity.
In another study Gazel looked at the chemical composition of volcanic rocks from global databases and integrated petrologically-constrained temperature and pressure of melts with seismic imaging data from around the world. In this way, Gazel and his colleagues determined that there is a prevalent, previously unknown layer of melt below the Earth’s tectonic plates. This study has given researchers a better understanding of the internal dynamics of the planet and of a boundary layer that plays a critical role in the formation of volcanoes, and thus the atmosphere and nutrient-rich continental crusts.
Like Holycross, Gazel has also been involved in the rare-earth mineral and exoplanet research projects mentioned earlier. Their expertise and technical innovations have allowed researchers in fields as seemingly-distinct as rare-earth mineral extraction and planetary exploration to make significant strides.
Cool it
Where Holycross and Gazel are focused most intently on geologic processes happening at great depth and high heat, their colleague Assistant Professor Nicole Fernandez keeps her work on the surface and at much lower temperatures. Rocks formed under great pressure and heat sometimes make it to the surface of the Earth, and when they do, they inevitably fall apart. Earth—that great re-user and recycler—wears them away and finds new ways to put them to work.
Fernandez is a low temperature hydrogeochemist. She studies the interactions between fluids and rocks at or near the surface. What we see and experience as the Earth’s surface is largely the result of hydrologic and biogeochemical processes and Fernandez wants to understand these processes better.
As Fernandez explains on her lab group’s website, she calls her lab the CritChem research group because “our group has a primary interest in the geochemistry of terrestrial systems, which comprise the most vibrant and vulnerable portion of the Earth’s surface – the Critical Zone.” She combines laboratory experimentation, field work, and numerical modeling to characterize the wide range of water-rock interactions and their role in element recycling, water quality, soil production, climate change, net-zero energy production, and the evolution of the Earth system.
One way to examine water-rock and plant-water-rock interactions on the Earth’s surface is through the use of geochemical tools such as stable isotopes and trace element environmental tracers, but to better leverage them we need to fully understand how they behave. A major focus of her work is to expand the toolkit hydrogeochemists have at their disposal, developing tracers and numerical models researchers can then apply to many aspects of field work across both space- and timescales.
Before coming to Cornell, Fernandez collaborated with a group of researchers in France to improve a method for accurately measuring waterborne isotopes of silicon using a multi-collector plasma mass spectrometer. One application of these efforts has been an investigation of the extent to which extreme storm events offer insight into watershed transport and biogeochemical relationships through river silicon isotope signatures of watersheds from a diversity of locations: France, the Caribbean, British Columbia, California, and New Mexico. Fernandez is currently applying her expertise in watershed (bio)geochemistry closer to home in local streams like Cascadilla and Fall Creek.

This past summer, while collecting water samples from Cascadilla Creek and rainwater samples from the roof of Snee Hall, Fernandez and her team of students uncovered a surprising result: both the stream and the rainwater samples showed marked spikes in phosphorus concentration. Phosphorus is an important nutrient for freshwater life and can drive harmful algal blooms in lakes and ponds. After some investigation it became clear that the phosphorus spikes were a result of the ongoing presence of wildfire smoke from Canada over the Finger Lakes region of New York. If Fernandez’s team had not been collecting daily samples from Cascadilla Creek and rainwater samples from Snee Hall, the connection between wildfire smoke and elevated phosphorus content in the water might have been missed.
Another thread of Fernandez’s work is the development of a regional 3D scale groundwater and reactive transport model for Aquitaine Basin—which is the second-largest river basin in France.
This work is being done in collaboration with several French scientific organizations. This basin has been inhabited by humans for a long time and has been heavily impacted by both farming and urban development. Fernandez and colleagues hope to combine several forms of tracer concentration data with a numerical model to, in effect, rewind time and create a history of recharge events covering the past 40,000 years.
Fernandez and EAS Professor Lou Derry are working to integrate groundwater ages with watershed chemistry through a new NSF award to use groundwater and chemical tracers to disentangle relationships between how long water spends in the subsurface and water quality in a small catchment, Sagehen Creek, situated in the Central Sierra Mountains near Lake Tahoe.
Over time she will factor in the effects of dust inputs in tropical areas, urban runoff and hidden streams in heavily developed areas, and the influence of plants in all areas.
Fernandez, Gazel, and Holycross have added new life to the study of geochemistry at Cornell’s Department of Earth and Atmospheric Sciences. Each in their own way is filling in the details behind the mechanisms that drive the building up, the wearing away, and the recycling of the very stuff that makes the Earth.

